What You Need To Know About Energy
The future of American society and its unprecedented standard of living depend, to a large degree, on how we use energy. The energy choices we make shape not only our quality of life, but the health of the environment, how we work and play, the strength of our economy, and our national security. Sound decisions by individuals, communities, and the nation depend on trustworthy and objective energy information. To help fill that need, the National Academies of Sciences, Engineering, and Medicine provide this energy primer.
Uses
Discover how the strength of American industry, speed of transportation, and countless modern conveniences all come from our ingenious use of energy.
Sources
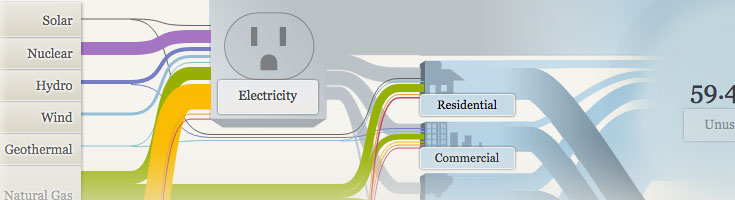
The United States depends on a variety of energy sources. What advantages and challenges does each one present to our nation and its people?
Costs
Learn about the costs of our high standard of living—to the environment, to our national security, and to irreplaceable resources.
Efficiency
Increasing supply isn’t the only answer to a stable energy future. Discover how reducing demand through improved efficiency achieves the same effect.
Explore Other Topics
Energy Defined
- Corporate Average Fuel Economy (CAFE)
Federal standards that stipulate a target average fuel economy rating (typically expressed in miles per gallon, or mpg) to be met by passenger vehicles by a certain date. The most recent version of the CAFE law, pending passage by Congress, requires new cars, SUVs, and light trucks to average 35.5 mpg by 2016.
Source Material
- America’s Energy Future: Technology and Transformation (2009)
- Real Prospects for Energy Efficiency in the United States (2010)
- Electricity from Renewable Resources: Status, Prospects, and Impediments (2010)
- Liquid Transportation Fuels from Coal and Biomass: Technological Status, Costs, and Environmental Impacts (2009)